
Amphiphilic silicone-bridged bis-triazoles as effective, selective metal ligands and biologically active agents in lipophilic environment
Pairs of different substituted 3-mercapto-1,2,4-triazole units are coupled, through thioether bridges, to organic-inorganic substrates consisting in short hydrophobic silicone segment. A library of six compounds are isolated as crystalline solids and structurally characterized by X-ray single crystal diffraction, elemental, spectral and thermal analysis. The flexibility of the silicone spacer makes the small molecular compounds exhibit glass transition in the negative domain. The metal binding capacity is evaluated by quantum mechanics calculations, the results being in line with experimental data obtained by UV–vis spectroscopy titration. The results indicate that the prepared compounds can act as ligands for metal ions with high selectivity for Cu2+, an element of interest in biological processes, forming 1:1 stable mononuclear complexes with an association constant up to 8.87 × 103 M−1. The presence of the highly hydrophobic silicone spacer makes the behavior of bis-triazoles obtained more sensitive to the nature of the environment. The preliminary bioassay indicates lipophilic medium more suitable for biocide action of silicone-bridged bis-triazoles, which in some cases far exceeds that of reference. The mechanism of enzyme inhibition is demonstrated by molecular docking, and the results indicate that, in all docked complexes, the ligands are directly coordinated to the heme ferric iron.

Copper complexes with spherical morphology generated in one step by amphiphilic ligands: in situ view of the self-assembling, characterization, catalytic activity
Cyclotri- and tetrasiloxanes having a methyl group and a carboxylic acid coupled through thioether bridge at each silicon atom were used to coordinate copper(II). The amphiphilic character conferred by the co-existence of the two moieties with opposite behaviour on the silicon atom gives the molecule the ability to form aggregates in solution. When copper salt is added, the metal is quickly coordinated by carboxyl groups within these aggregates, as in situ taken microscopy images and video reveal, forming blue spheres. This morphology was confirmed by scanning and transmission electron microscopy, while the coordinating structures and their compositions were estimated by IR spectroscopy and UV–Vis diffuse reflectance spectroscopy, wide angle X-ray diffraction and energy-dispersive X-ray analysis. The wetting tests and moisture sorption analysis indicate hydrophobic materials, while thermal analysis shows a thermal stability of at least up to 180 °C. The compounds catalyze the decomposition of H2O2 in alkaline medium, the values found for the rate constant being between 0.8 and 3.8x10-3s-1.

Linear and cyclic siloxanes functionalized with polar groups by thiol-ene addition: Synthesis, characterization and exploring some material behaviour
1,3-divinyltetramethyldisiloxane (V2), 1,3,5-trivinyl-1,3,5-trimethylcyclotrisiloxane (V3), and 1,3,5,7-tetravinyl-1,3,5,7-tetramethylcyclotetrasiloxane (V4) are used as hydrophobic substrates to attach carboxyl groups by thermal or photochemical activated thiol-ene addition of 3-mercaptopropionic acid (R1), thioglycolic acid (R2) and thioacetic acid (R3). The reactions occurrence is monitored by IR following the disappearance of specific absorption bands for -S-H and -CH=CH2 bonds. At the end, the structure of the compounds and thioetherification degree of vinyl groups are determined by NMR. Co-existence in the structure of the highly hydrophobic methyl group and the carboxyl or carbonyl group gives the compounds formed an amphiphilic character, as indicated by the calculated hydrophilic-lipophilic balance, making them capable of self-assembling in the solution, as the results of the dynamic light scattering (DLS) and nanoparticle tracking analysis (NTA) measurements also show. Under normal temperature conditions, these compounds are in the liquid state. The DSC measurements in the range (-150) – (+150) reveal only a glass transition in the negative field and no other thermal transitions or decomposition processes. This behavior could make these compounds containing polar groups useful as free-solvent liquid electrolytes. Dielectric spectra reveal an increase in both dielectric permittivity and conductivity of the thiol-ene addition products as compared with starting vinyl-siloxanes. The increase is even more significant when the formers are doped with lithium, reaching the conductivity of order 10-4 S/cm.
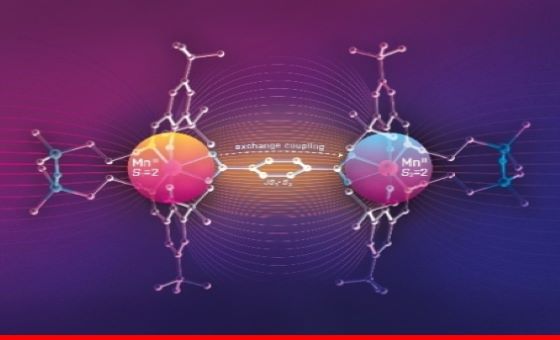
Dinuclear manganese(III) complexes with bioinspired coordination and variable linkers showing weak exchange effects: a synthetic, structural, spectroscopic and computation study
Three dimanganese(III) complexes have been synthesised and fully characterised by standard spectroscopic methods and spectroelectrochemistry. Each MnIII ion is chelated by a salen type ligand (H2L), but there is variation in the bridging group: LMn(OOCCH=CHCOO)MnL, LMn(OOCC6H4COO)MnL, and LMn(OOCC6H4C6H4COO)MnL. X-ray diffraction revealed an axial compression of each six-coordinate high-spin d4 MnIII ion, which is a Jahn-Teller-active ion. Temperature dependent magnetic susceptibility and variable temperature-variable field (VTVH) magnetisation measurements, as well as high-frequency and -field EPR (HFEPR) spectroscopy were used to accurately describe the magnetic properties of the complexes, not only the single-ion spin Hamiltonian parameters: g-values and zero-field splitting (ZFS) parameters D and E, but also the exchange interaction constant J between the two ions, which has been seldom determined for a di-MnIII complex, particularly when there is more than a single bridging atom. Quantum chemical calculations reproduced well the electronic and geometric structure of these unusual complexes, and, in particular, their electronic absorption spectra along with the spin Hamiltonian and exchange parameters.

Hydrophobic, amorphous metal-organic network, readily prepared by complexing the aluminium ion with a siloxane spaced dicarboxylic acid in aqueous medium
A less common dicarboxylic acid with siloxane spacer, 1,3-bis(carboxypropyl)tetramethyldisiloxane, whose structure is for the first time determined crystallographically, is used to coordinate to aluminium through an environment-friendly preparation process, resulting in a polymeric structure of amorphous metal-organic framework (aMOF) type. The high flexibility of the spacer induced by the siloxane bond gives the polymeric product a glass transition slightly below room temperature, while the long length of the former creates the premises for collapse of the network and consequently reduced porosity. At the same time, the high hydrophobicity of the tetramethyldisiloxane fragment and its low surface energy, that causes it to migrate to the air interface, gives the network low moisture sorption capacity guaranteeing the stability of its properties in a wet environment. The particulate complex, of the order of one to two hundred nanometers, as generated by the synthesis, proves to be suitable as filler for silicone with outstanding reinforcement effect, without significantly affecting their transparency. The presence in the structure of dimethylsiloxane, which also underlies the structure of the matrix, ensures a good incorporation of the filler without the need for special compatibility treatments.

Metallo-supramolecular assemblies of dinuclear Zn(II) and Mn(II) secondary building units (SBUs) and a bent silicon dicarboxylate ligand
Two metallo-supramolecular polymers {[Zn2(L)4(DMF)2]·0.8DMF}n (1) and {[Mn2L2(DMSO)4]·2DMSO}n (2) (H2L = bis(p-carboxyphenyl)diphenylsilane) have been synthetized by the reaction of Zn(NO3)2·6H2O and Mn(NO3)2·4H2O respectively, with the above mentioned acid as ligand under solvothermal conditions. Both coordination polymers were structurally characterized by single crystal X-ray diffraction, elemental analysis, FTIR and UV–Vis spectroscopy. Single crystal X-ray diffraction analysis reveals dinuclear Zn(II) and Mn(II) nodes bridged by mono or double deprotonated ligand molecules coordinated in syn-syn bidentate mode. Thermal and moisture stability and photophysical properties of the resulting coordination polymers were studied and correlated with their structure.

Keto-enol tautomerism in new silatranes Schiff bases tailed with different substituted salicylic aldehyde
New Schiff base-type products starting from 1-(3-aminopropyl)silatrane and three derivatives of salicylaldehyde having as substituents 3,5-dichloro- (1), 3-methoxy- (2) and 3,5-di-tert-butyl- (3), respectively were obtained and isolated with high yields (78-87%) in pure, crystalline forms and their structures were established by different methods. The molecular electronic transitions of the compounds in solvents with various polarities were investigated by UV-Vis spectral analysis. Their thermal behavior was studied by thermogravimetric analysis and differential scanning calorimmetry, results of the latter highlighting thermocromism of the compounds proved by the appearance of IR absorption bands specific for enolic form at temperature corresponding to each sample. The moisture sorption capacity and stability of the compounds in wet environment were investigated by vapor sorption analysis in dynamic regime and IR spectroscopy. The biological activity was assessed by specific tests. All results were discussed in correlation with the nature of substituents and structures formed. The chemical handling of the silatrane tail, by using different substituents on the silicon atom would allow fine tuning of the compounds properties.

Silver thin films generated by Pulsed Laser Deposition on plasma-treated surface of silicones to get dielectric elastomer transducers
TA protocol to monitor the condensation crosslinking process of a polydimethylsiloxane-α,ω-diol –based films with tetraethoxysilane in the presence of an organometallic compound (dibutyltindilaurate, DBTDL) was established using IR spectroscopy as an economical and noninvasive analysis technique. Under the presence of the environmental humidity, the hydrolysis of the ethoxysilane occurs with the formation of highly reactive Si-OH groups, which easily condense with similar ones or/and with the Si-OH on the ends of the PDMS chains leading to their crosslinking and dimensional stabilization of the film. In absolutely anhydrous systems, the reaction does not occur, or occurs only very slowly. The evolved by-products, alcohol and water, slowly diffuse out of the hydrophobic film. In fact, this reaction, which is also referred to curing or vulcanization, starts with the formation of a skin at the film surface that is in direct contact with environmental moisture and continues gradually towards inside, involving a curing time that depends on the environmental humidity, film thickness, temperature and catalyst amount...

A new bis(μ-chlorido) bridged cobalt(II) complex with silyl-containing Schiff-base as catalyst precursor in solvent-free oxidation of cyclohexane
A new bis(µ-chlorido)-bridged cobalt(II) complex [Co2(µ-Cl)2(HL2)4][CoCl4] (1), where HL2 is a silyl-containing Schiff base, was synthesised. The structure of this compound was established by X-ray crystallography revealing a zwitterionic form adopted by the organic ligand. The temperature dependence of the magnetic susceptibility and the field dependence of the magnetisation indicate the presence of ferromagnetic interactions between paramagnetic d7 cobalt(II) centres (SCo = 3/2). The exchange coupling parameter J(Co1–Co2) = +7.0 cm–1 extracted from broken-symmetry (BS) DFT calculations agrees well with the value of +8.8 cm–1 determined from the experimental data by fitting them with the Hamiltonian math formula. Electrochemical studies indicate that complex 1 is inefficient as a catalyst in electrochemical reduction of protons. One of the reasons is the low stability of the complex in solution. In contrast, 1 acts as an effective homogeneous (pre)catalyst in the microwave-assisted neat oxidation of cyclohexane with aqueous tBuOOH (TBHP). The possible mechanism of catalytic oxidation and other advantages of using 1 in the oxidation of cycloalkanes are discussed.

A five-coordinate manganese(III) complex of a salen type ligand with a positive axial anisotropy parameter D
A new high-spin d4 roughly trigonal-bipyramidal (TBP) manganese(iii) complex with a salen type ligand (H2L), namely MnL(NCS)·0.4H2O, has been synthesised and characterised by elemental analysis, ESI mass spectrometry, IR and UV-vis spectroscopy, and spectroelectrochemistry. X-ray diffraction analysis revealed an axial compression of the approximate TBP. Temperature dependent magnetic susceptibility and variable-temperature variable-field (VTVH) magnetisation measurements, as well as high-frequency and -field EPR (HFEPR) spectroscopy, were used to accurately describe the magnetic properties of this complex and, in particular, determine the spin Hamiltonian parameters: g-values and the zero-field splitting (ZFS) parameters D and E. The HFEPR spectra allowed the extraction of fourth order ZFS parameters. Quantum chemical calculations reproduced well the electronic and geometric structures of this unusual complex and, in particular, its electronic absorption spectrum along with the spin Hamiltonian parameters.

Chapter: Aminosilicones as Active Compounds in the Detection and Capture of Co2 from the Environment.
Because anthropogenic carbon dioxide emissions in the atmosphere have greenhouse effect contributing to global warming, worldwide efforts are being made to reduce them, developing techniques for separating and capturing CO2 being a priority. One of the most effective technologies for CO2 capture consists of chemical absorption in a liquid medium containing the amine (alkanolamine, ammonia) with the formation of carbamate or bicarbonate. Because the reaction is reversible, CO2 can then be removed by heating with the amine regeneration and reuse it. Silicone materials have also been studied as means of capturing CO2, among them amino-silicones recently proved to be highly efficient absorber of this. For such use, amines containing siloxanes has several advantages over the classic organic amines, such as high thermal stability, low volatility and low viscosity, which allows their use as such, without the need for dissolution/dilution with water or organic solvents. This makes the heat energy needed to release CO2 and absorber regeneration to be reduced. The effectiveness of the amino-silicones in retaining CO2 is extended in their use as sensors for this gas. This chapter critically reviewed and analyzed the results of the authors and those reported in the literature on both these directions.

4th International Conference on Chemical Engineering (ICCE 2018) Iasi, Romania, October 31 - November 2, 2018
Our team participated at the 4th International Conference on Chemical Engineering (ICCE 2018) - Innovative materials and processes for a sustainable development that took place in Iasi, Romania between October 31 - November 2, 2018, at “Gheorghe Asachi” Technical University of Iași, Romania. The ICCE 2018 is co-organized by “Gheorghe Asachi” Technical University of Iasi, “Cristofor Simionescu” Faculty of Chemical Engineering and Environmental Protection and "Gheorghe Asachi" Universitary Foundation from Iași, under the auspices of Romanian Academy and supported by Iași City Hall and industrial partners. The Conference occurs every two years being as a reference for researchers and students for the high standard presentations in the field. The ICCE 2018 included three days of a scientific and social program with keynote lectures, oral and poster presentations. During the Conference, the participants had the opportunity to present their research work, made new contacts and networking, shared experience. Our team was represented by Mirela-Fernanda Zaltariov and participated with one communication: “Homogeneous and heterogeneous catalysts with silane/siloxane-based discrete metal complexes and metal-organic frameworks”.

The XXXVth Romanian Chemistry Conference,Călimăneşti – Căciulata, Romania, October 02 - 05, 2018
Our team participated at The XXXVth Romanian Chemistry Conference that took place in Călimăneşti – Căciulata, Romania between October 02-05 2018. The aim of the Conference is to review the state of the art and to explore the ways in which basic and applied researches in various fields of chemistry interact. Our team was represented by Angelica Vlad and Alexandra Bargan at the poster session where they presented the posters : “Strategii de obtinere a retelelor metal-organice (MOFs) pe baza de liganzi de tip carboxilat cu siliciu” and respectively “Metal complexes of a siloxane ligand derived from pyrrole 2-carbaldehyde”.

12th International Conference on Physics of Advanced Materials (ICPAM-12) Heraklion, Crete, Greece, September 22 - 28, 2018
Our team participated at the 12th International Conference on Physics of Advanced Materials (ICPAM-12) that took place in Heraklion, Crete, Greece between 22-28 September 2018. The events were organized by Alexandru Ioan Cuza University and Technological Educational Institute of Crete, together with prestigious universities and research institutes all around the world. The main objective of ICPAM-12 was to provide a forum for scientists involved in physics of advanced materials, emerging nanotechnologies and new devices, to exchange their knowledge and experience. The ICPAM-12 sessions referred to recent developments and new strategies and included the basic invitation, oral and poster presentations that cover a wide range of hot interdisciplinary research domains. Our team was represented by Georgiana-Oana Turcan-Trofin at the poster session where she presented a poster : “Copper coordination compounds with spherical morphology based on methylcyclosiloxanes functionalized with carboxyl groups as ligands”

Tenth Cristofor I. Simionescu Symposium Frontiers in Macromolecular and Supramolecular Science, Romanian Academy, Bucharest, June 8 – 14, 2018
The Romanian Academy, the "Petru Poni"Institute of Macromolecular Chemistry in Iasi, the Laboratory for Material Structure Research (LRSM) of the University of Pennsylvania (USA) and the National Scenical Foundation - NSF (USA) organized the tenth edition of the International Symposium "Cristofor I. Simionescu" – Frontiers in Macromolecular and Supramolecular Science.This symposium was created in 2008 to celebrate the life and achievements of Professor Christofor I. Simionescu, who was the founding father of Macromolecular Science in Romania and the Director of the ’’Petru Poni’’ Institute of Macromolecular Chemistry in Iasi, Romania, for 30 years (1970–2000). Participating plenary speakers have included the leaders of macromolecular science from the USA, Europe, and Asia.
This year the Symposium took place in Bucharest, at the Romanian Academy during 8 and 14 june 2018 and two members of our team participated with one conference – Mirela-Fernanda Zaltariov ’’Versatility of silane/siloxane building blocks in coordination driven self-assembling’’- and one short communication – Georgiana-Oana Turcan-Trofin ’’New ligands and materials developed on silicone substrates’’.

The 21st International Conference on Solid Compounds of Transition Elements, SCTE18, Vienna, March 25 - 29, 2018
Between 25 and 29 March 2018 our team participated at the 21st International Conference on Solid Compounds of Transition Elements, SCTE'2018 that which took place in Vienna, Austria. The SCTE 2018 follows a series of conferences, dating back more than 50 years, with recent meetings in Annecy, France, (2010), Lisboa, Portugal (2012), Genova, Italy, (2014) and Zaragossa, Spain (2016). The SCTE has always been a forum where new ideas and discoveries, related to the solid state chemistry and solid state physics of d- and f-element compounds are presented and discussed. The conference deals with the structure, crystal chemistry, chemical bonding, and magnetic, and electronic transport properties of different classes of intermetallic compounds. Fundamental and applied research in the areas of solid state chemistry, physics and materials science of compounds were included. Our team was represented by Mirela-Fernanda Zaltariov and Georgiana-Oana Turcan-Trofin at the poster session where each presented a poster : ’’Lanthanide-based MOFs built on silicon-containing carboxylate ligands: Synthetic strategies and properties evaluation’’ and ’’Functionalization of siloxane derivatives by attaching polar groups’’ respectively.

A XXVIII-a ediție a Congresului Internațional „Pregătim viitorul promovând excelența”,Apollonia, Iasi, Romania, March 1 - 4, 2018
Between 1 and 4th of March our team participated at the Congresului Internațional „Pregătim viitorul promovând excelența” that took place in Iasi, Romania with the oral communication "New silicone derivatives with biocidal activity" presented by Georgiana-Oana Turcan-Trofin.


















